X Chromosome Inactivation: Unlocking Genetic Disease Treatments
X chromosome inactivation is a fascinating biological process that is crucial for understanding genetic diseases impacting women, such as Fragile X Syndrome and Rett Syndrome. This unique mechanism ensures that females, who possess two X chromosomes, do not produce double the amount of proteins encoded by the genes located on these chromosomes compared to males. The advent of research surrounding the X chromosome has unveiled critical insights, particularly the role of the Xist RNA molecule, which orchestrates the silencing of one of the two X chromosomes. This chromosomal therapy approach may pave the way for effective treatments for various X-linked disorders. As researchers continue to delve into the complexities of X chromosome inactivation, the potential for breakthroughs in alleviating the burden of genetic diseases becomes increasingly tangible.
The phenomenon of X chromosome silencing plays a pivotal role in the regulation of gene expression, especially in females who possess an additional X chromosome compared to their male counterparts. Known as X-inactivation, this natural process enables equalization in gene dosage between the sexes, thereby preventing the overexpression of X-linked genes. The emergence of therapies targeting conditions such as Fragile X Syndrome or Rett Syndrome showcases the innovative strides scientists are making in understanding genetic regulation. A key player in this process is the Xist RNA molecule, which is instrumental in modulating the chromatin structure necessary for effective chromosomal therapy. As we explore the intricacies of X chromosome silencing, the horizon looks promising for developing solutions to overcome genetic challenges.
Understanding X Chromosome Inactivation and Its Role in Genetic Diseases
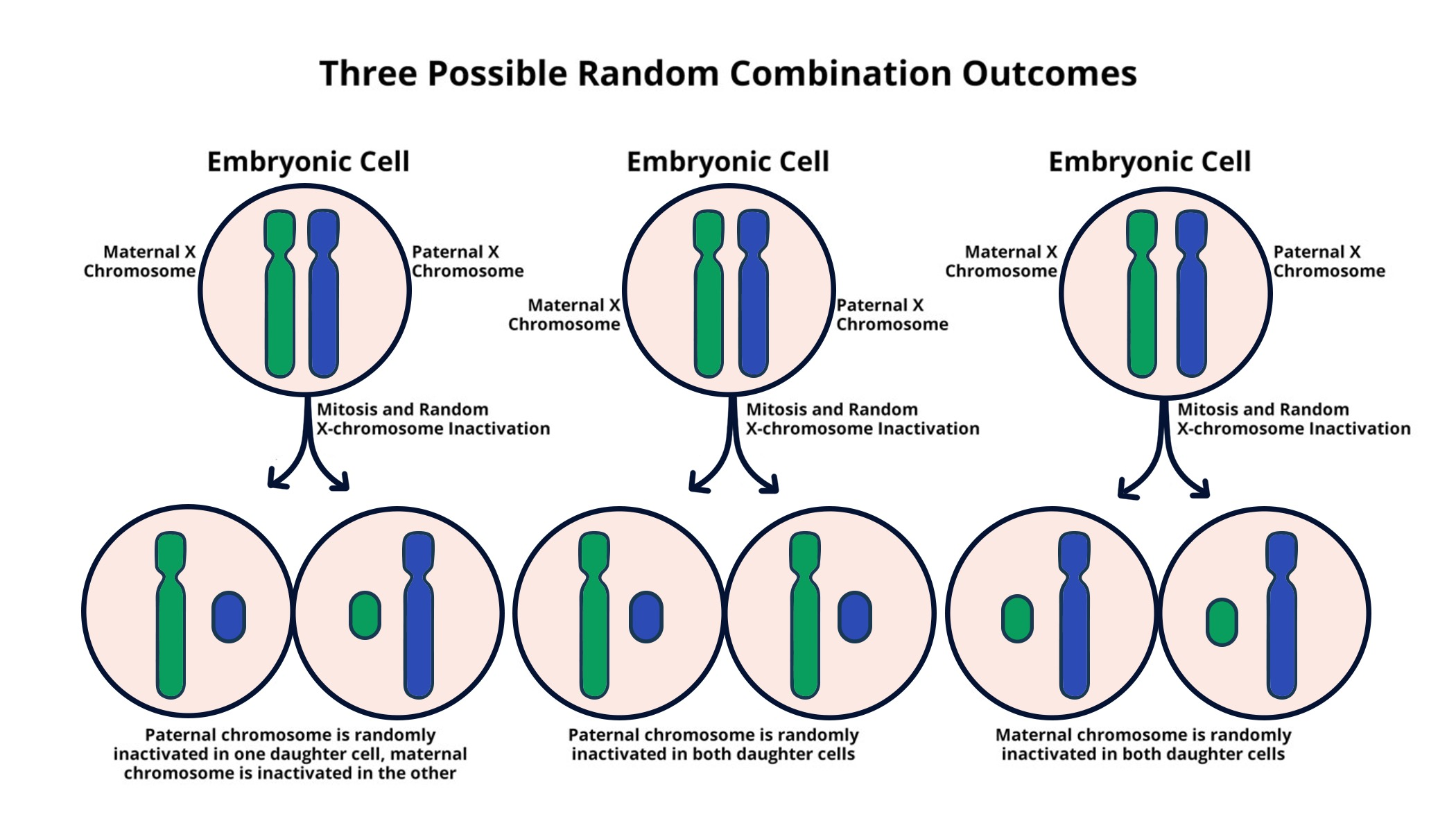
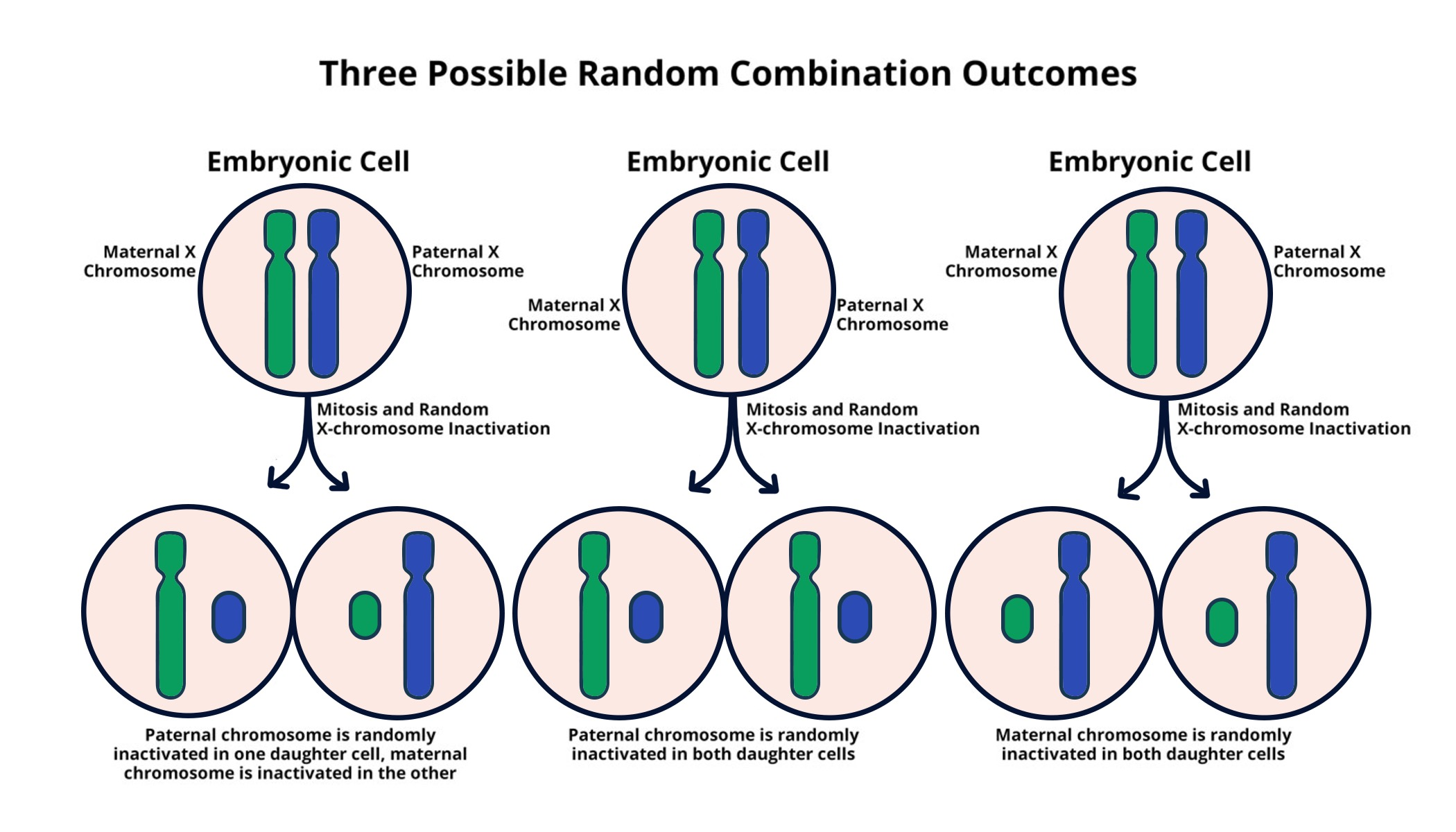
X chromosome inactivation (XCI) is a crucial biological mechanism that ensures dosage compensation in female mammals, balancing the expression of X-linked genes between males and females. This complex process is vital for normal development and functioning, as it enables females, who have two X chromosomes, to inactivate one, preventing an overdose of genes that can lead to various genetic disorders. The intricacies of XCI, particularly how the Xist RNA molecule plays a central role in silencing one of the X chromosomes, have fascinated researchers for decades. Understanding the mechanisms of XCI not only sheds light on fundamental biology but also has direct implications for tackling genetic diseases linked to X chromosome abnormalities, such as Fragile X Syndrome and Rett Syndrome.
The discovery of Xist’s action at the X chromosome has paved the way for potential therapies targeting genetic diseases. Recent advances in chromosomal therapy leverage the understanding of XCI processes, aiming to reactivate silenced genes. This approach is promising for conditions such as Fragile X Syndrome, where a mutation on one of the X chromosomes leads to intellectual disabilities. Similarly, Rett Syndrome involves mutations in the MECP2 gene located on the X chromosome. By unsilencing specific genes through the manipulation of XCI, researchers are hopeful that they can restore normal function and alleviate the symptoms of these challenging disorders. Therefore, unraveling the mechanics of XCI is not only essential for genetic research but also offers new avenues for treatment development.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases like Fragile X Syndrome?
X chromosome inactivation is a biological process that occurs in females, where one of the two copies of the X chromosome is randomly inactivated to prevent an overdose of X-linked gene products. This process is crucial for understanding genetic diseases such as Fragile X Syndrome because it can influence how symptoms manifest in individuals and how certain therapies might be developed to reactivate the silenced chromosome.
How does the Xist RNA molecule contribute to X chromosome inactivation?
The Xist RNA molecule is essential for X chromosome inactivation. It is produced by the inactivated X chromosome and coats the chromosome, changing the surrounding chromosomal material properties, which facilitates silencing. Understanding the function of Xist is vital for developing treatments for disorders such as Rett Syndrome, where X-inactivation plays a significant role.
What potential therapies are being researched for Fragile X Syndrome that involve X chromosome inactivation?
Research on X chromosome inactivation has revealed potential therapies that may unsilence the inactivated X chromosome, allowing access to healthy gene copies that can alleviate the symptoms of Fragile X Syndrome. These therapies aim to leverage the mechanisms of Xist RNA and other molecules responsible for chromosomal silencing.
How does X chromosome inactivation provide insights into treatments for Rett Syndrome?
The study of X chromosome inactivation offers insights into Rett Syndrome as it primarily affects females, leading to the hypothesis that reversing inactivation of the healthy X chromosome could restore function to mutations responsible for the disorder. This possibility highlights the role of Xist in exploring gene therapy options.
Can chromosomal therapy effectively target genes affected by X chromosome inactivation?
Chromosomal therapy aims to target genes affected by X chromosome inactivation, especially for disorders like Fragile X Syndrome and Rett Syndrome. By potentially ‘unsilencing’ genes on the inactivated chromosome, researchers hope to enable the expression of healthy versions of genes that are currently inactive due to this biological process.
What are the challenges in understanding X chromosome inactivation related to genetic diseases?
One of the challenges in understanding X chromosome inactivation in relation to genetic diseases is distinguishing the impact of mutations on gene expression. For instance, while X chromosome inactivation might silence mutated genes associated with disorders like Fragile X Syndrome, identifying why unaffected genes remain functional poses complexity in developing targeted therapies.
Is it possible to reverse X chromosome inactivation for therapeutic benefits in genetic diseases?
The concept of reversing X chromosome inactivation presents a promising therapeutic avenue, particularly for genetic diseases. Research has shown that it may be possible to ‘unsilence’ an inactivated X chromosome, thereby allowing for the expression of healthy genes and offering new treatment strategies for conditions like Fragile X Syndrome and Rett Syndrome.
| Key Points |
|---|
| Females have two X chromosomes, but only one is functionally active due to X chromosome inactivation. |
| X chromosome inactivation occurs through a process that silences one of the two X chromosomes in females, ensuring equal gene expression between sexes. |
| Xist, an RNA molecule, plays a crucial role in initiating the inactivation process by modifying the surrounding chromatin structure. |
| The study hints at potential therapies for genetic disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. |
| Ongoing research aims to optimize methods to reactivate inactivated X chromosomes, paving the way for clinical trials. |
| Mysteries remain regarding how reactivating inactivated X chromosomes can restore function to mutated genes while sparing healthy ones. |
Summary
X chromosome inactivation is a critical biological process that ensures females, possessing two X chromosomes, maintain balanced gene expression similar to males who have one. Recent research led by Jeannie T. Lee’s lab reveals the complexities of this process, highlighting the role of Xist RNA and a gelatinous substance that modifies the chromatin environment, enabling efficient silencing of one X chromosome. The implications of this work are significant, as it opens avenues for therapies targeting genetic diseases linked to the X chromosome, specifically Fragile X and Rett syndromes. By exploring the mechanisms of reactivating inactivated X chromosomes, researchers hope to develop effective treatments, potentially transforming outcomes for individuals affected by these conditions.