X-Chromosome Inactivation: A Breakthrough for Genetic Disorders
X-chromosome inactivation is a fascinating and complex process that plays a crucial role in the genetic landscape of female mammals. Unlike males, who possess a single X chromosome, females have two, leading to the need for one of these X chromosomes to be silenced to ensure cellular balance. This notorious silencing mechanism has captivated researchers for decades, particularly in understanding its implications for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Recent breakthroughs in this field have illuminated how X chromosomes are inactivated, uncovering potential avenues for treatment of X-linked mutations. With a focus on these genetic challenges, scientists are optimistic that advancements in understanding X-chromosome inactivation will pave the way for innovative therapies that could alleviate the burden of these conditions.
The phenomenon of X-chromosome inactivation, often referred to as X-inactivation, serves as a critical biological equalizer in females, ensuring that gene dosage between sexes is balanced. This intriguing process not only plays a pivotal role in normal cellular function but also has significant implications for understanding various inherited disorders, such as Fragile X Syndrome and Rett Syndrome. By investigating how one of the two X chromosomes in females becomes inactive, researchers are uncovering vital insights that could lead to breakthroughs in the treatment of genetic disorders linked to X-linked mutations. This chromosomal silencing acts as both a safeguard and a challenge, as it can complicate the expression of potentially beneficial genes that are trapped in the inactive state. As studies continue to unfold, the potential for therapeutic strategies based on X-chromosome inactivation becomes increasingly promising for those affected by these genetic complexities.
Understanding X-Chromosome Inactivation
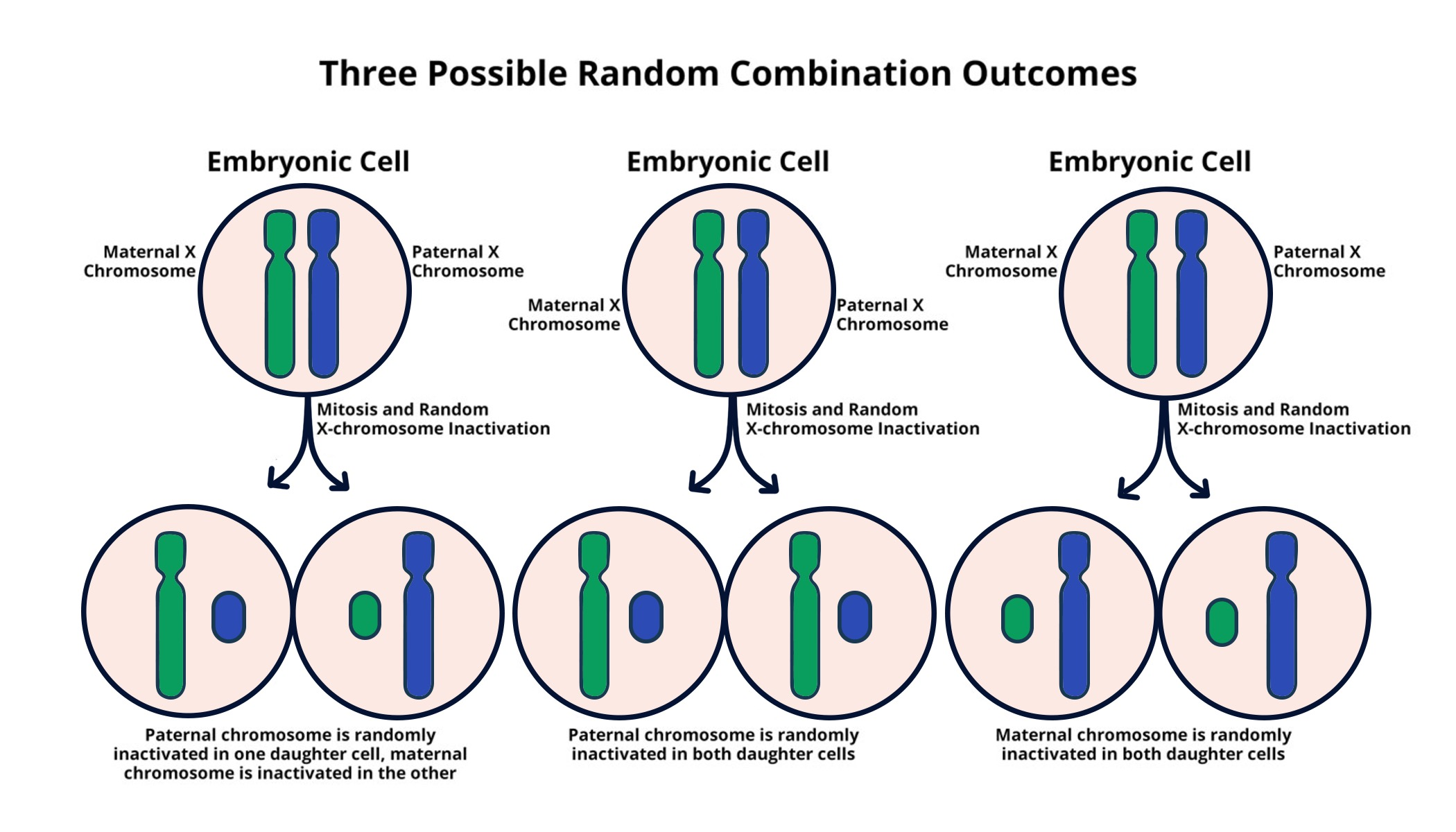
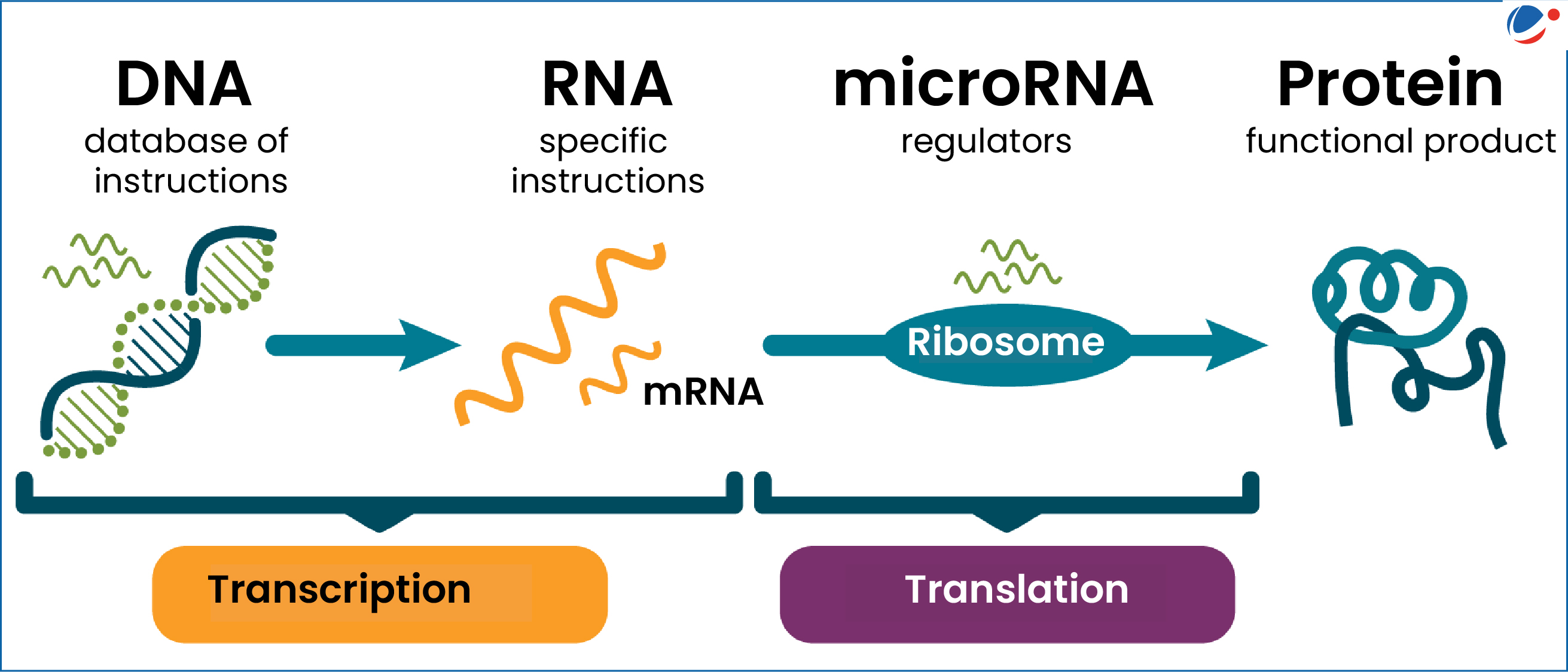
X-chromosome inactivation (XCI) is a crucial biological process that allows female mammals, who possess two X chromosomes, to balance the gene dosage between males and females. This mechanism ensures that only one of the X chromosomes in females is active while the other is silenced, preventing an overload of gene expression. The process, characterized by the intricate interactions of various molecular players, has significant implications for understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome, which are caused by mutations on the X chromosome. By exploring the dynamics of XCI, researchers like Jeannie T. Lee have begun to unravel the complexities that underlie this fundamental process, which may lead to innovative therapeutic approaches to these genetic disorders.
In her recent groundbreaking research, Lee’s team discovered that a gelatinous substance surrounding the chromosomes plays a critical role in X-inactivation. This viscous material, which resembles Jell-O, aids in separating chromosomes to prevent tangling and can influence the behavior of molecules like Xist, which initiates the inactivation of one of the X chromosomes. As Xist interacts with this Jell-O-like substance, it alters its properties, promoting an environment conducive to silencing the chromosome. Understanding how XCI functions at a molecular level is not only a triumph of basic scientific inquiry but also a potential gateway to overcoming the challenges posed by X-linked mutations in treating related disorders.
Implications of X-Chromosome Inactivation for Genetic Disorders
The implications of X-chromosome inactivation extend beyond basic biology, particularly concerning genetic disorders such as Fragile X Syndrome and Rett Syndrome. Both conditions are characterized by mutations that predominantly affect the X chromosome. By harnessing the mechanism of XCI, scientists are exploring the possibility of reactivating the healthy genes that are silenced due to the inactivation process. For instance, the recent advances in unsilencing X-linked genes could pave the way for targeted therapies aimed at restoring normal gene function in individuals affected by these disorders. As highlighted by Lee, this potential therapeutic strategy could revolutionize treatment and provide relief for thousands of patients, marking a significant shift in the landscape of genetic disorder management.
Moreover, the findings surrounding X-chromosome inactivation have broader implications for our understanding of gene expression in both males and females. While males possess only one X chromosome, certain X-linked mutations can still lead to significant health issues, as in the case of Fragile X Syndrome. The research indicates that the biological mechanisms instructing XCI may also regulate the expression of specific genes, providing insights that could lead to targeted interventions. By unlocking the secrets of XCI, researchers are not only addressing the challenges associated with X-linked mutations but may also be setting the stage for a new era of treatment for various genetic disorders.
Research Breakthroughs in X-Chromosome Dynamics
Decades of research into the dynamics of X-chromosome inactivation have culminated in recent breakthroughs that illuminate how this complex process functions. Researchers like Jeannie Lee have utilized advanced techniques to delve deep into the molecular interactions at play, leading to discoveries that hold significant promise for treating genetic disorders. The journey from understanding a fundamental biological question to applying this knowledge therapeutically reflects the progression from basic science to clinical application – a hallmark of successful research in genetics. This evolution of understanding is crucial not only for scientific advancement but also for developing new treatment modalities for X-linked conditions such as Fragile X Syndrome.
Among the most exciting revelations is the role of the Jell-O-like substance that surrounds chromosomes, which has been shown to facilitate the inactivation process. By making the surrounding medium more flexible and accessible, researchers can exploit these properties to develop methods for unsilencing inactivated genes. This could lead to innovative therapies that target specific gene mutations, especially in conditions where only one copy of the gene is healthy, such as in Fragile X Syndrome. The potential to free up these inactivated X chromosomes not only offers hope for improved treatments but also exemplifies the significance of chromosomal breakthroughs in understanding and addressing genetic disorders.
Future Directions in Gene Therapy for X-Linked Disorders
The promising advancements in understanding X-chromosome inactivation have opened up new avenues for gene therapy aimed at treating X-linked disorders. As researchers like Jeannie Lee refine their techniques for unsilencing genes on the inactivated X chromosome, the potential for developing targeted therapies becomes increasingly viable. Current strategies involve sophisticated methodologies that aim to maximize the accessibility of these genes, especially in conditions like Fragile X Syndrome and Rett Syndrome, where mutations hinder normal function. The goal is to restore not only gene activity but also to improve the overall quality of life for affected individuals.
Looking ahead, the field of genetic therapy will likely see a surge in clinical trials that investigate the efficacy and safety of these innovative treatments. By optimizing the existing approaches and conducting thorough safety studies, researchers aim to translate laboratory findings into practical applications that can benefit patients. Furthermore, the insights gained from studying X-inactivation may well extend beyond X-linked disorders, influencing therapeutic strategies for a wider array of genetic conditions. As research progresses, the focus will remain on harnessing these breakthroughs to develop solutions that improve patient outcomes and advance the field of genetics.
The Role of RNA Molecules in X-Chromosome Inactivation
Central to the X-chromosome inactivation process is the role of RNA molecules, particularly Xist. This RNA molecule is pivotal in the silencing of one X chromosome in females. Its production marks the initiation of a series of events that ultimately lead to the inactivation of the chromosome, ensuring that gene dosage is balanced between the sexes. The fascinating tug-of-war between Xist and the gel-like substance that encapsulates each chromosome not only highlights the complexity of epigenetic regulation but also serves as a critical function in gene expression management. Understanding this interaction may yield further insights into related genetic disorders.
Moreover, the ongoing research into how Xist interacts with its environment has important implications for therapeutic interventions. As findings suggest that Xist can be manipulated to unsilence genes, particularly those affected by mutations causing conditions like Fragile X Syndrome, the potential for RNA-based therapies emerges. Such therapies could precisely target and reactivate silenced genes, paving the way for effective treatments. With continued research in the dynamics of RNA interactions and chromosome behavior, the future holds exciting possibilities for leveraging these molecules in the fight against genetic disorders.
Exploring Chromosomal Mechanisms Behind Fragile X and Rett Syndromes
Both Fragile X Syndrome and Rett Syndrome are deeply rooted in the molecular intricacies of the X chromosome. Fragile X Syndrome, linked to mutations in the FMR1 gene on the X chromosome, leads to cognitive impairment and developmental delays, while Rett Syndrome, caused by mutations in the MECP2 gene, results in severe neurological impairments. The challenges presented by these conditions underscore the necessity for comprehensive research into the cellular mechanisms underlying them. By decoding the complexities of X-chromosome inactivation, researchers are uncovering critical insights that could lead to breakthroughs in treatment.
Investigating the cellular pathways involved in X-inactivation not only sheds light on the specific mutations responsible for both syndromes but also helps identify potential therapeutic targets. If scientists can successfully develop strategies to unsilence the healthy gene variant bound within the inactive X chromosome, there may be a chance to alleviate some of the debilitating symptoms associated with these disorders. The exploration of chromosomal mechanisms is thus not merely an academic exercise; it holds tangible potential for translating scientific discoveries into effective clinical outcomes for those affected by these challenging genetic disorders.
The Genetics of X-Linked Disorders: A New Perspective
The landscape of genetic disorders is continually evolving, particularly as we enhance our understanding of X-linked conditions. The research conducted by Jeannie T. Lee and her colleagues has provided a new perspective on how X-chromosome inactivation affects the expression of genes associated with conditions like Fragile X Syndrome and Rett Syndrome. By highlighting the complexities surrounding the inactivation mechanism and its impact on gene expression, their work invites a re-examination of therapeutic approaches aimed at managing X-linked disorders. This shift in perspective emphasizes the necessity of innovative strategies that target the underlying genetic mechanisms rather than merely addressing symptoms.
With advancements in genetic engineering and molecular biology, the potential for developing targeted therapies is promising. Researchers are increasingly exploring methods that can selectively reactivate genes silenced through XCI, offering hope for individuals affected by such disorders. Furthermore, the insights gained from these studies may have critical implications for the treatment of a variety of genetic conditions beyond those associated with X-linked mutations. The new perspectives on the genetics of X-linked disorders represent not only a scientific breakthrough but also an essential step toward enhancing patient care and therapeutic efficacy.
Ethical Considerations in Gene Therapy for Genetic Disorders
As research progresses toward potential gene therapies for conditions like Fragile X and Rett Syndromes, it raises critical ethical considerations that must be addressed. The manipulation of genetic material, especially in the context of X-chromosome inactivation, poses questions about the long-term effects and societal implications of such treatments. The prospect of reactivating genes within inactivated X chromosomes necessitates a thorough consideration of potential unintended consequences on gene expression, genetic diversity, and the ethical ramifications of ‘editing’ the human genome. Engaging in comprehensive ethical discussions will be vital as the field transitions from basic research to clinical applications.
Moreover, ethical guidelines will need to evolve alongside advances in genomic technologies. As scientists explore the potential to develop therapies that could dramatically alter the lives of those afflicted by X-linked disorders, it is essential to ensure that these interventions are safe, equitable, and accessible. The conversation surrounding ethical considerations in gene therapy should encompass the perspectives of patients, families, and the broader community impacted by genetic disorders, fostering a dialogue that prioritizes informed consent, the welfare of future generations, and the responsible use of biotechnological advancements.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important for understanding genetic disorders like Fragile X Syndrome?
X-chromosome inactivation is a biological process where one of the two copies of the X chromosome in females becomes inactive, which helps balance gene dosage between males (who have one X) and females. This process is crucial for understanding genetic disorders such as Fragile X Syndrome, as it can affect the expression of genes responsible for these disorders, leading to potential insights into treatment options.
How does X-chromosome inactivation relate to Rett Syndrome?
Rett Syndrome is an X-linked neurodevelopmental disorder primarily affecting females. Understanding X-chromosome inactivation is vital for researching Rett Syndrome because it can highlight how mutations on the X chromosome lead to the disease and how therapies might reactivate silenced genes to alleviate symptoms.
Can X-chromosome inactivation techniques be used to treat X-linked mutations in genetic disorders?
Yes, techniques aimed at reactivating inactivated X chromosomes may serve as potential therapies for genetic disorders caused by X-linked mutations, such as Fragile X Syndrome and Rett Syndrome. Research indicates that unsilencing these genes can restore their function, potentially providing a pathway to treat these disorders.
What recent advancements have been made in understanding X-chromosome inactivation’s role in chromosomal breakthroughs?
Recent advancements in understanding X-chromosome inactivation involve discovering how RNA molecules like Xist interact with the chromatin structure, akin to a gelatinous substance. This understanding could lead to therapeutic strategies to treat various genetic disorders linked to X-linked mutations by reactivating beneficial genes.
How could breakthroughs in X-chromosome inactivation research impact male patients with conditions like Fragile X Syndrome?
Breakthroughs in X-chromosome inactivation research could impact male patients with conditions like Fragile X Syndrome by identifying mechanisms to target and silence or unsilence specific genes on the X chromosome. Even though males have only one X, similar regulatory mechanisms may allow for targeted treatments that can alleviate symptoms.
What role does the substance described as ‘Jell-O’ play in X-chromosome inactivation?
The ‘Jell-O-like’ substance acts to separate chromosomes within the cell, enabling X-chromosome inactivation to occur. It helps facilitate the action of molecules like Xist, which changes the properties of this substance, allowing for efficient silencing of genes on the inactive X chromosome.
In what ways can understanding the mechanisms of X-chromosome inactivation lead to innovative therapies for genetic disorders?
By understanding the mechanisms of X-chromosome inactivation, researchers can develop innovative therapies aimed at unsilencing genes that may be mutated on the inactivated X chromosome, providing new treatment avenues for disorders like Fragile X and Rett Syndromes, ultimately improving patient outcomes.
What future prospects are there for therapies targeting X-chromosome inactivation?
Future prospects for therapies targeting X-chromosome inactivation include transitioning from basic research to clinical trials, potentially developing treatments that specifically reverse the effects of mutations on the X chromosome without adversely affecting healthy genes, which could transform care for those with genetic disorders.
| Key Points |
|---|
| Human cells face a challenge with the X chromosome since females have two copies and males have one, necessitating X-inactivation in females. |
| A gene called Xist plays a critical role in the X-inactivation process, interacting with a gelatinous substance that coats chromosomes. |
| Jeannie T. Lee’s research reveals that Xist changes the material properties of the surrounding substance, allowing access for other crucial molecules to facilitate inactivation. |
| This chromosomal silencing could eventually lead to treatments for disorders like Fragile X Syndrome and Rett Syndrome, as it enables access to healthy genes that were previously inactivated. |
| Current research aims at optimizing techniques to ‘unsilence’ X-linked genes, with hopes to begin clinical trials in the near future. |
| Interestingly, treatments may benefit males as well since similar silencing mechanisms affect mutated genes on the X chromosome. |
Summary
X-chromosome inactivation is a critical cellular process that ensures females, who possess two X chromosomes, only express genes from one. This natural phenomenon poses intricate challenges for gene expression, especially in the context of genetic disorders linked to the X chromosome. Recent research led by Jeannie T. Lee has unveiled the mechanisms by which cells enact this inactivation, primarily through the action of the Xist gene and its interaction with a gelatinous substance surrounding chromosomes. The implications of understanding X-chromosome inactivation are profound, offering hope for targeted therapies for conditions like Fragile X and Rett syndromes, which have traditionally been challenging to manage.